
Difference Between Covalent and Ionic Bonds Gcse Chemistry Revision
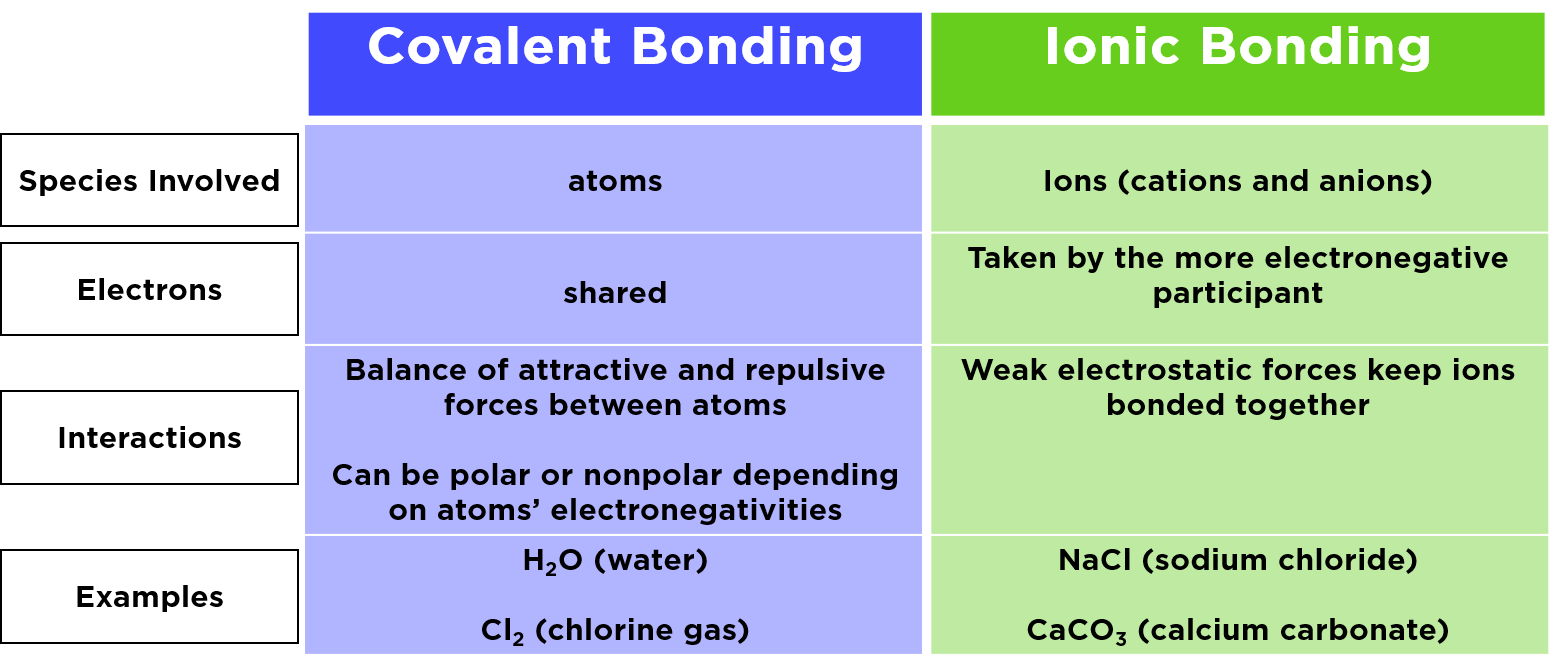
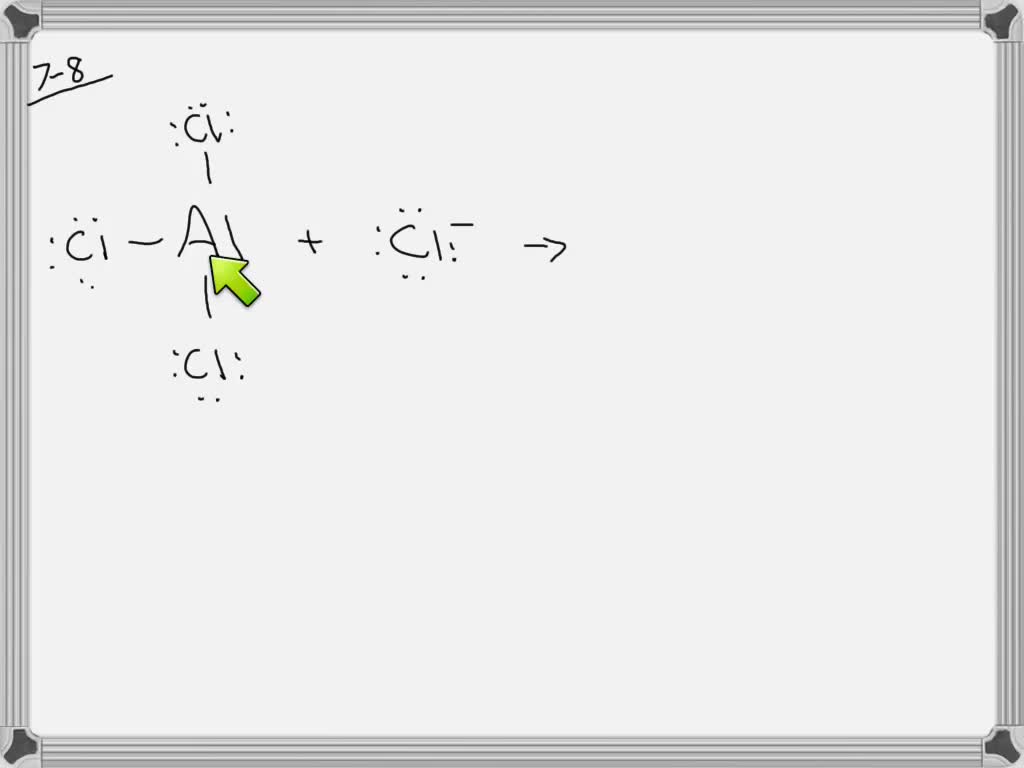
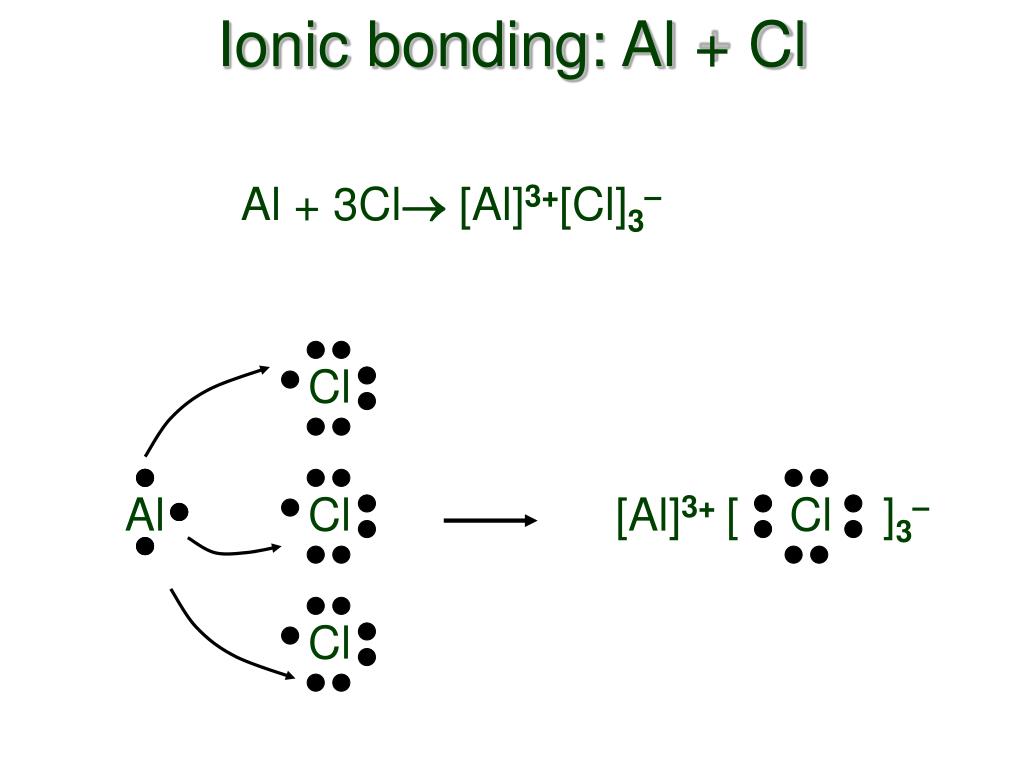
AlCl3 is an ionic compound because it contains an aluminum cation (Al3+) and a chloride anion (Cl-). The Al3+ ion has a charge of +3, while the Cl- ion has a charge of -1. This creates an unequal distribution of electrons between the two atoms, which forms an ionic bond. What Are Ionic And Covalent Bonds?

How to Draw the Lewis Structure for AlCl3 Aluminum Chloride YouTube
The Al-Cl bond in AlCl₃ is not ionic — it is polar covalent. The electronegativity of Al is 1.5. The electronegativity of Cl is 3.0. So ΔEN = 1.5. This corresponds to a bond that is 57 % covalent and 43 % ionic. Molten AlCl₃ is a poor conductor of electricity, unlike NaCl. In the gas phase, AlCl₃ is a trigonal planar molecule, analogous to BCl₃.
Ionic Bond — Formation & Compounds Expii
Figure2.7.3 2.7. 3: Example of some compounds that have multiple oxidation states. Note mercury (1) is not a monoatomic cation, but is really a homonuclear diatomic ion of two mercury atoms bound to each other, both having lost one electron. So Hg 2 Cl 2 it the lowest whole number ratio of cation to anion. Example 2.

Ionic Bond Definition, Types, Properties & Examples
Sodium chloride and magnesium chloride are ionic and consist of giant ionic lattices at room temperature Aluminium chloride and phosphorus (V) chloride are tricky! They change their structure from ionic to covalent when the solid turns to a liquid or vapour. There is much more about this later on this page. The others are simple covalent molecules.

Is AlCl3 ionic or covalent or both? What type of bond in Aluminium
Ionic and molecular compounds are named using somewhat-different methods. Binary ionic compounds typically consist of a metal and a nonmetal. The name of the metal is written first, followed by the name of the nonmetal with its ending changed to - ide. For example, K 2 O is called potassium oxide.
KWOK The Chem Teacher Chemical Bonding Dative Covalent and Expansion
1 By Fajans' rules, we can easily find out which compound shows covalent character. Example: Among NaCl N a C l, MgClX2 M g C l X 2, AlClX3 A l C l X 3 which one is more covalent? Answer: There is a point in Fajans' rules that the compound which has more charge number that compound will show covalent character.

Is Alcl3 Ionic Or Covalent? Breaking Down The Basics
Therefore AlF3 is ionic but AlCl3 is covalent. Electronegativity difference:- If Electronegativity difference between bounding atoms is less than 1.7 then bond has more covalent charactor. And Electronegativity difference is 1.55 between Al and Cl. Therefore Al——Cl bond is covalent. Suggest Corrections.

Is AlCl3 Ionic or Covalent? Techiescientist
Is Alcl3 Ionic or Covalent? View Solution Click here:point_up_2:to get an answer to your question :writing_hand:alcl3 is covalent where as alf3 is ionic why

Anhydrous AlCl3 is covalent but hydrated AlCl3.6H2O is ionic because
Is Alcl3 Ionic or Covalent? Leave a Comment / Chemistry / By reta Chemical bonds are the forces that hold atoms together to form molecules. There are two main types of chemical bonds - ionic and covalent bonds.
Anhydrous AlCl3 is covalent if the energy to ionize AlCl3 = 5215 kJ mol
1 Answer Sorted by: 6 The distinction between ionic and covalent compounds is arbitrary and many compounds fall somewhere in the middle. This is explained in a previous question. In the case of AlClX3 A l C l X 3 we have a metal 'cation' and a non-metal 'anion' so in a simplistic view we would expect this to be an ionic compound.

AlCl3 Molecular Geometry Science Education and Tutorials
Compounds that contain ions are called ionic compounds. Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules (uncharged groups of atoms that behave as a single unit), are called covalent compounds.

Leave a Comment Cancel Reply
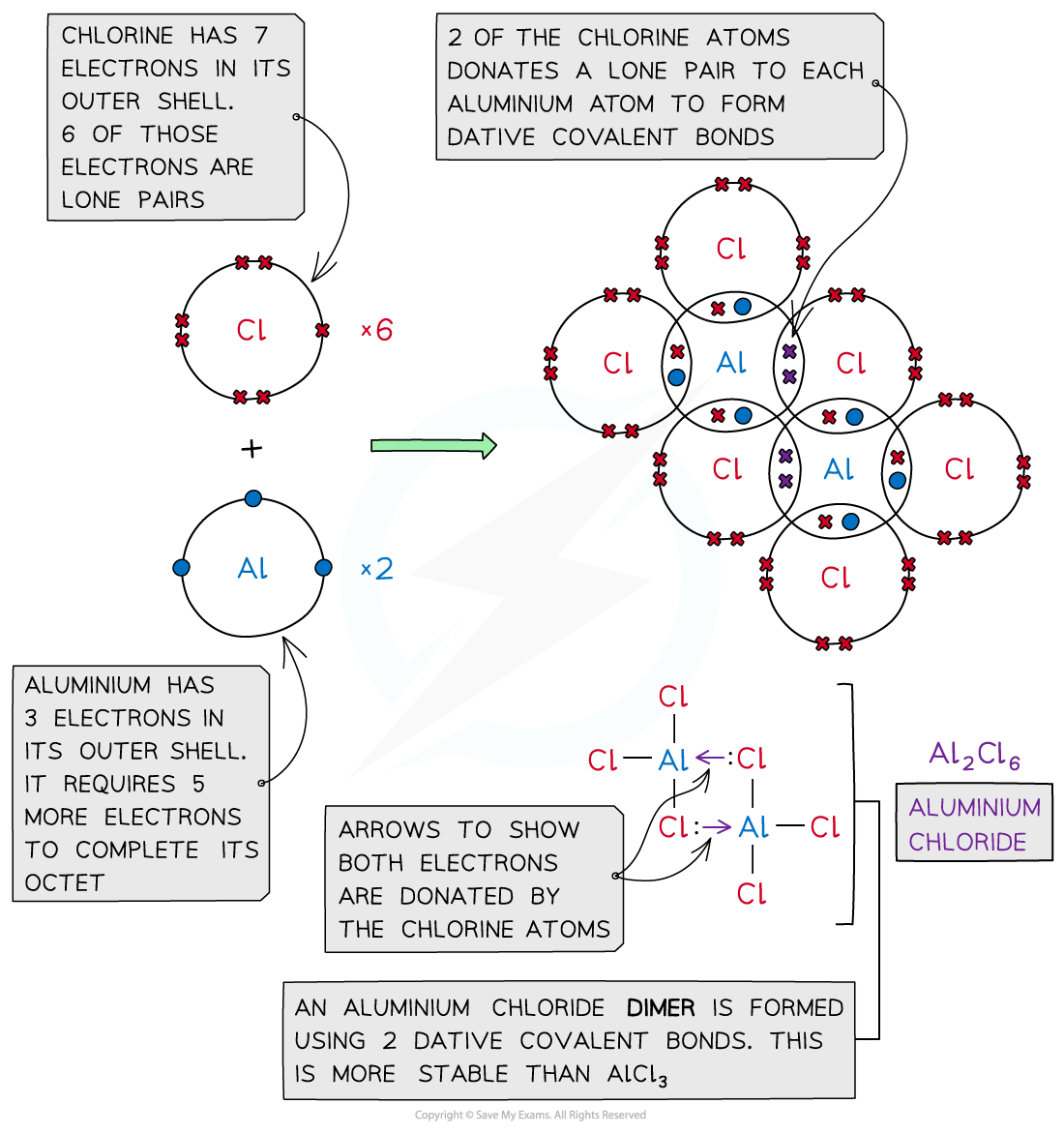
Why does AlCl3 form a dimer? AlCl3 is borderline for being ionic/covalent. When solid it is usually ionic. In vapor it is covalent. In this state 3 electrons from Al are shared with 3 Cl electrons.

Which of the following is a covalent compound?
AlCl3 is not ionic, but a covalent compound. Because aluminum and three chlorine atoms are linked through the sharing of electrons with each other, although, aluminum (Al) is a metal and chlorine (Cl) is a nonmetal, still, there does not occur a complete transfer of electrons between the metal and the non-metal.
SOLVEDAluminum chloride, AlCl3, behaves more as a molecular compound
Is AlCl3 Ionic or Covalent? Let us study the nature of an antiperspirant compound known as aluminum chloride. Aluminum chloride is also identified as aluminum trichloride. The formula of this compound is AlCl3 (H2O)n (n = 0 or 6). It has a molecular weight of 133.34. Anhydrous aluminum chloride is a white to gray powder that smells pungent.
PPT Bonding, Electronegativity , & Bond Shape PowerPoint Presentation
1 OH group is smaller than Cl and Al (OH)3 isn't molecular, unlike the chloride. - Mithoron Jun 28, 2022 at 19:39 In the pure state, |AlClX3 | A l C l X 3 dimerizes as a molecule AlX2ClX6 A l X 2 C l X 6. The structure is made of two squares having one edge in common (like an open book).
Edexcel A Level Chemistry复习笔记1.4.6 Covalent DotandCross Diagrams翰林国际教育
Aluminum chloride is an inorganic compound. AlCl3 is an odorless and white/ pale yellow crystalline corrosive solid. It exists in a hydrated (AlCl3.6H2O) and an anhydrous form (AlCl3). In this article, we will understand the concepts of prediction of Lewis structure, geometry, hybridization, and polarity of a given compound. Contents show